Acids bases & ph worksheet answer key – Embark on an enlightening journey into the fascinating realm of acids, bases, and pH with our comprehensive worksheet answer key. This definitive guide unveils the fundamental concepts, properties, calculations, applications, and safety considerations surrounding these essential chemical entities, empowering you with a profound understanding of their significance in various scientific disciplines and everyday life.
Delve into the intricacies of acid-base reactions, unravel the mysteries of pH measurement, and discover the myriad industrial, household, and environmental implications of these remarkable substances. Prepare to illuminate your path through the complexities of acids, bases, and pH, leaving no stone unturned in your quest for knowledge.
Acids, Bases, and pH Definitions
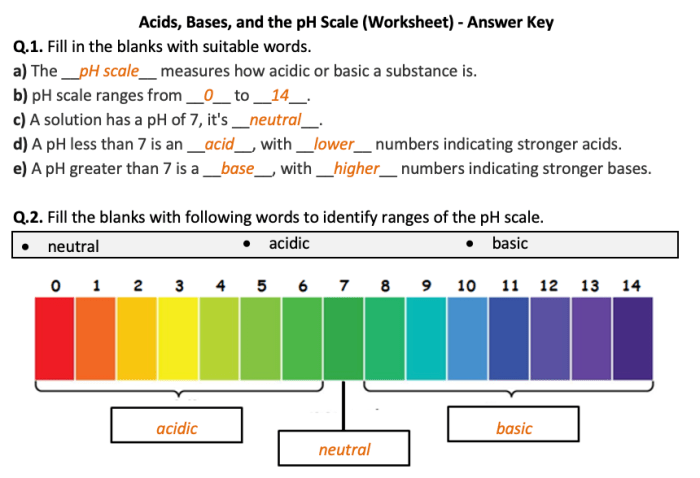
Acids are chemical compounds that release hydrogen ions (H+) in a water solution, while bases are compounds that release hydroxide ions (OH-) in water. The pH scale measures the acidity or alkalinity of a solution on a scale from 0 to 14, with 0 being the most acidic and 14 being the most basic (alkaline).
A pH of 7 is neutral.
Common examples of acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3). Common examples of bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)2).
Properties of Acids and Bases
Acids are generally sour to taste, corrosive to metals, and turn litmus paper red. Bases are bitter to taste, slippery to touch, and turn litmus paper blue. Acids and bases react with each other in a process called neutralization, which produces water and a salt.
Acids and bases play a crucial role in many chemical reactions, such as the formation of salts, the digestion of food, and the regulation of pH levels in biological systems.
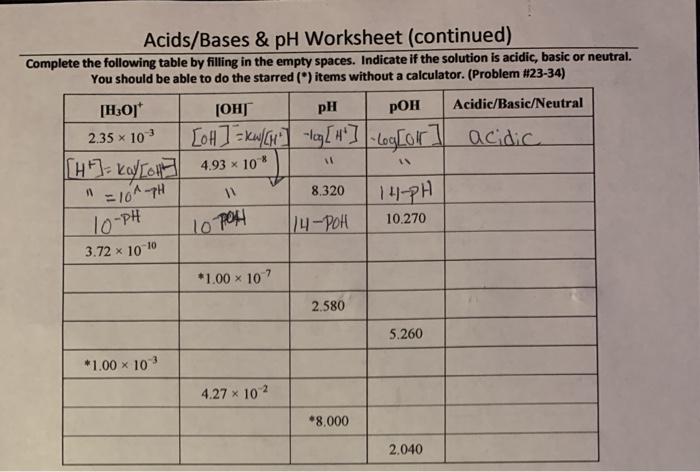
pH Calculations and Measurement
The pH of a solution can be calculated using the formula pH = -log[H+], where [H+] is the molar concentration of hydrogen ions in the solution. pH can also be measured using pH meters or indicators, which are substances that change color depending on the pH of the solution.
pH is an important parameter in various applications, such as environmental monitoring, industrial processes, and medical diagnostics.
Applications of Acids and Bases
Acids and bases have numerous industrial applications, including the production of fertilizers, plastics, and pharmaceuticals. They are also used in everyday products such as batteries, cleaning agents, and food preservatives.
However, acid and base pollution can have adverse environmental impacts, such as soil acidification, water pollution, and harm to aquatic life.
Safety Considerations, Acids bases & ph worksheet answer key
Acids and bases can be hazardous, causing skin burns, eye damage, and respiratory problems. It is important to handle them with care, wear appropriate personal protective equipment (PPE), and follow proper storage and disposal guidelines.
Popular Questions: Acids Bases & Ph Worksheet Answer Key
What is the pH scale?
The pH scale is a measure of the acidity or basicity of a solution, ranging from 0 to 14. A pH of 7 is neutral, while values below 7 indicate acidity and values above 7 indicate basicity.
How do I calculate the pH of a solution?
The pH of a solution can be calculated using the formula pH = -log[H+], where [H+] is the molar concentration of hydrogen ions in the solution.
What are some common acids and bases?
Common acids include hydrochloric acid (HCl), sulfuric acid (H2SO4), and nitric acid (HNO3). Common bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)2).