Name each of the following alkenes: a task that requires precision, clarity, and an understanding of the IUPAC rules governing alkene nomenclature. This guide delves into the intricacies of alkene naming, providing a systematic approach to accurately identify and classify these important organic compounds.
Alkenes, characterized by their carbon-carbon double bond, exhibit unique properties and play vital roles in various chemical processes. Understanding their nomenclature is essential for effective communication and comprehension within the scientific community.
Alkenes
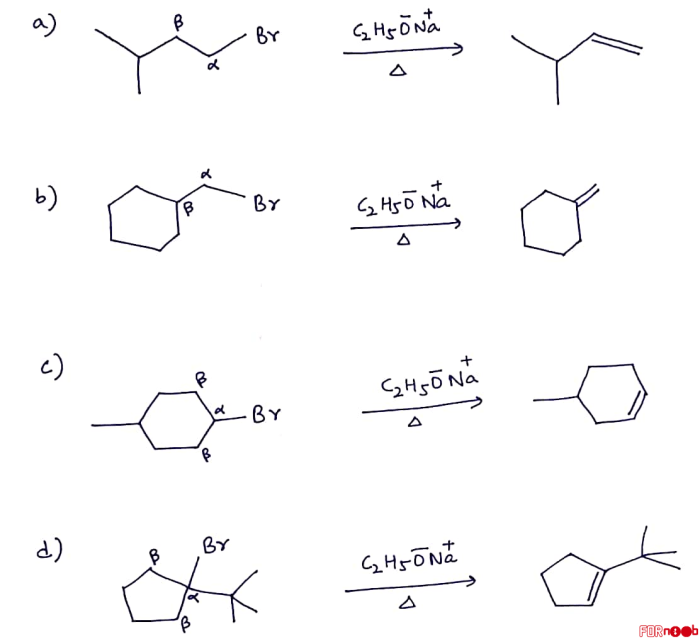
Alkenes are a class of unsaturated hydrocarbons that contain at least one carbon-carbon double bond. They have the general formula CnH2n, where n is the number of carbon atoms in the molecule. Alkenes are typically aliphatic, meaning that they have a chain-like structure.
They can be classified as either terminal or internal alkenes, depending on the location of the double bond.
Naming Alkenes: Name Each Of The Following Alkenes
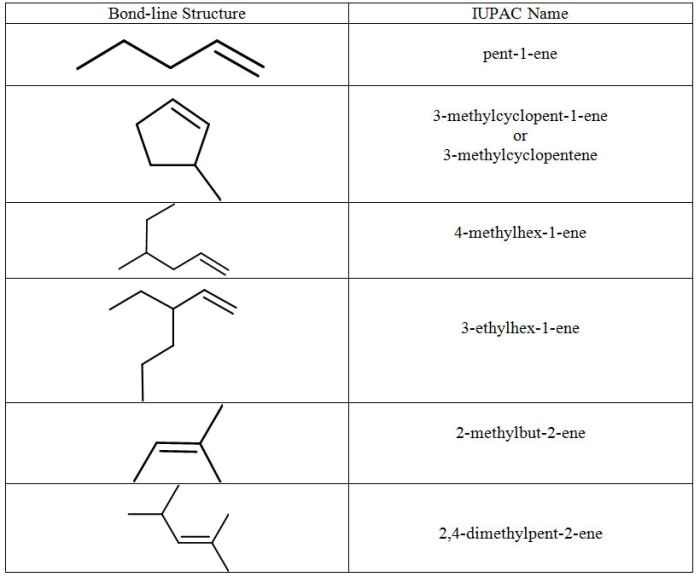
According to IUPAC rules, alkenes are named by adding the suffix “-ene” to the root name of the parent alkane. The root name is determined by the number of carbon atoms in the longest carbon chain that contains the double bond.
The location of the double bond is indicated by a number placed before the suffix. For example, the alkene with the molecular formula CH3CH=CHCH2CH3 is named 2-butene.
Structural Isomers of Alkenes, Name each of the following alkenes
Structural isomers are compounds that have the same molecular formula but different structural formulas. Alkenes can exhibit structural isomerism due to the different possible locations of the double bond along the carbon chain. For example, the alkene with the molecular formula C4H8 has two structural isomers: 1-butene and 2-butene.
These isomers have different physical and chemical properties.
Physical and Chemical Properties of Alkenes
Alkenes are typically nonpolar, low-boiling point liquids or gases at room temperature. Their boiling points increase with increasing molecular weight. Alkenes are more reactive than alkanes due to the presence of the double bond. They can undergo a variety of reactions, including addition, oxidation, and polymerization.
Applications of Alkenes
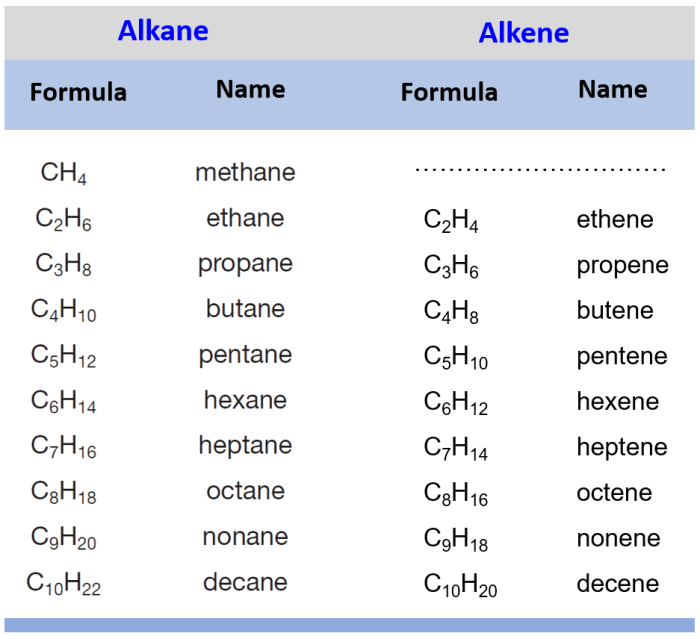
Alkenes are important industrial chemicals. They are used in the production of plastics, fuels, and other products. For example, ethylene is used to produce polyethylene, which is a common plastic. Alkenes can also be used as starting materials for the synthesis of more complex organic compounds.
Key Questions Answered
What is the significance of IUPAC rules in alkene nomenclature?
IUPAC rules provide a standardized system for naming alkenes, ensuring consistency and clarity in scientific communication. They establish guidelines for identifying the parent chain, numbering the carbon atoms, and incorporating prefixes and suffixes to accurately represent the structure of the alkene.
How do structural isomers affect alkene nomenclature?
Structural isomers of alkenes have the same molecular formula but different structural arrangements. IUPAC rules account for these variations by considering the position of the double bond and any substituents. The name of the alkene reflects the specific arrangement of atoms within the molecule.
What are the key considerations when naming substituted alkenes?
When naming substituted alkenes, it is crucial to identify and name the substituents correctly. Substituents are groups of atoms attached to the alkene chain. IUPAC rules specify the use of prefixes to indicate the type and position of substituents, ensuring precise identification of the compound.